Kinetic Theory
State of matter
Matter exists in the three states of Solid, Liquid and Gas. The physical difference between the three states of matter depends on the arrangement and behaviour of the molecules in each particular state.
This difference can be explained in terms of the Kinetic Theory, model which states that;
- Matter is made up of very small particles called molecules.
- These molecules are not stationary but are constantly moving.
- The degree of movement of the molecules depends on their temperature.
Solid
Properties of a solid substance
- Fixed shape and volume
- Normally hard and rigid
- Incompressible
- Large force needed to change shape
- High density
Arrangement and movement of particles
- The particles are close together.
- They are arranged in a regular pattern.
- The attractive forces between them are strong. The attractive force is called Cohesive force
- They vibrate to and fro at the fixed positions.
Liquid
Properties of a liquid
- Fixed volume but does not have a fixed shape
- Not compressible
- High density
Arrangement and movement of particles
- The particles are close together but they have wider space than those in solid.
- The attractive forces between them are weaker than those in solid.
- They move vigorously.
- They can move from one position to another.
Gas
Properties of gases
- No fixed shape or volume
- Compressible
- Low density
Arrangement and movement of particles
- The particles are very far apart.
- They can hardly attract each other.
- They can hardly attract each other.
Brownian motion
Brownian motion provides an evidence of the continuous random motion of the molecules in the air.
Diffusion
Diffusion is defined as the process by which different substances mix as a result of the randum motions of their molecules.
- The substances move freely from a region of high concentration to a region of low concentration at their own pace.
- The rate of diffusion depends on the temperature and the density of the substances involved.
- It supports the kinetic theory, since the particles must be moving to mix, and gases can be seen to diffuse faster than liquids.
Change of state
The names of changes of states are indicated in the diagram below.
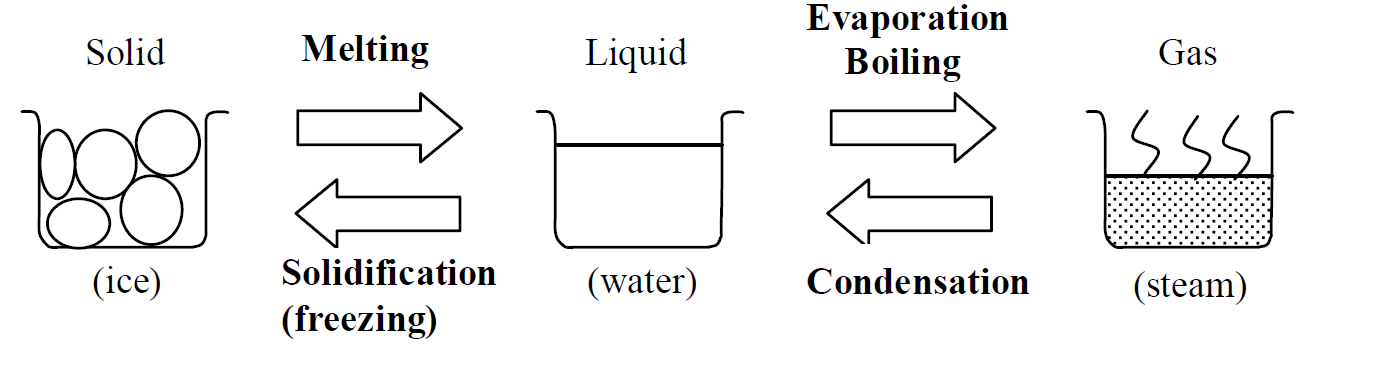
The temperature is unchanged during the change of state of a substance. All the energy supplied to the substance is used for breaking the strong forces between molecules.
- The melting point is the temperature at which a solid melts.
- The boiling point is the temperature at which a liquid boils.
Evaporation
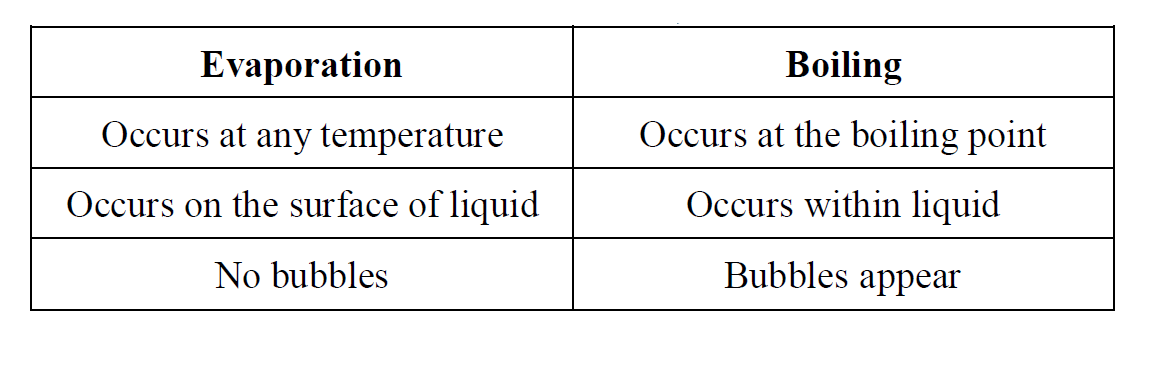
Evaporation is defined as the change of a liquid into a gas at the surface.
It occurs at any temperature but occurs more rapidly at higher temperature because heat gives more kinetic energy to the molecules and they escape from the surface faster.
Increased gas pressure on the surface of the liquid reduces the rate of evaporation because more collisions occur between the evaporating liquid molecules and the gas molecules, and some of the evaporated liquid molecules bounce back into the liquid.
The molecules that have the largest kinetic energy escape from the liquid. Then, the average kinetic energy of molecules in the liquid is reduced, and also the temperature of liquid reduces. This is called the cooling effect of evaporation.
The difference between evaporation and boiling