The Periodic Table
The periodic table is a chart of elements placed according to the order of increasing atomic numbers.
Atomic number is the property of elements used to place them in order on the periodic table.
Important features of the periodic table
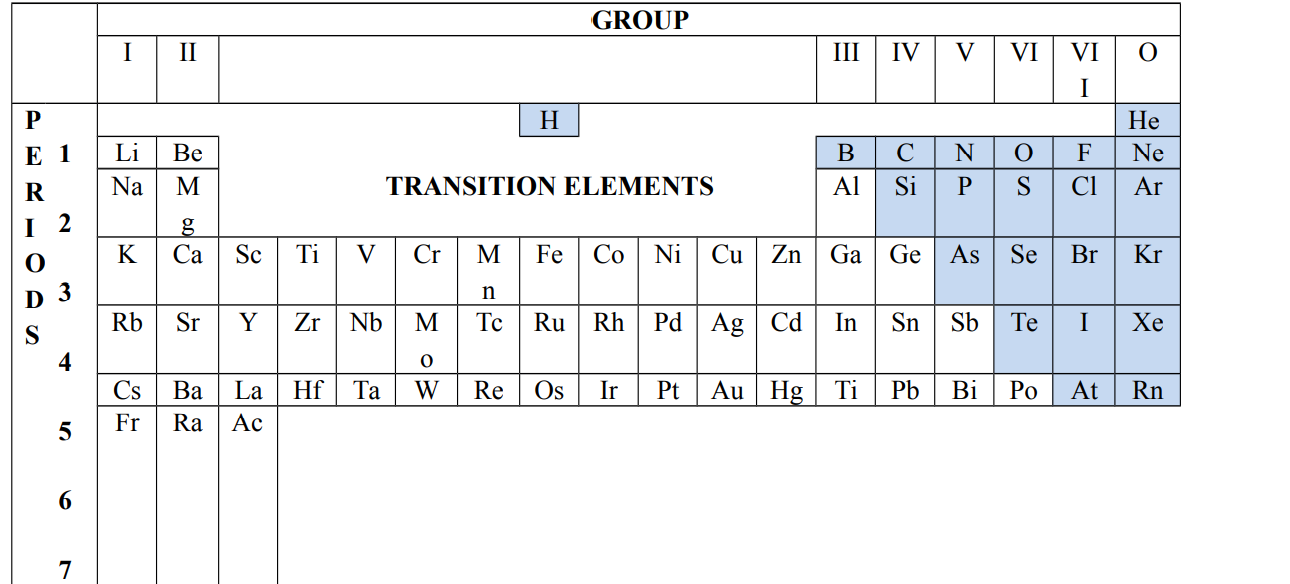
Things to note in the periodic table
- Elements are arranged in the order of increasing atomic numbers.
- Elements with similar electronic configuration are placed in the same group.
- Elements with similar chemical properties are placed in the same group
Period
A period is a horizontal row of elements in the periodic table
The periodic table has 7 periods.
- Period 1 contains two elements; hydrogen and helium
- Periods 2 and 3 contains eight elements each and are called short periods.
- Periods 4, 5 and 6 contain eighteen elements each and are called long periods.
- Period 7 contains three elements
Progression of properties across the period
As we move across the period (from left to right)
- The metallic nature of elements decreases. They change from metals to non-mental and inert gases.
- Electro-negativity increases. Electronegativity is the relative ability of an atom to attract the pair of electrons in a covalent bond.
- The atomic number increases by one between successive elements.
- The number of shells remains the same while the number of valence electrons increases steadily. The valence of each element is equal to;
Example 1
(a) The valence electrons for metals.
(i) Na 2. 8.1, valence = 1
(ii) Mg 2.8.2, valence = 2
(iii) Al 2.8.3, valence = 3
Example 2
(b) Eight (8) minus the valence electrons for non-metals
(i) Cl 2.8.7 Valency = 8 -7 = 1
(ii) O 2.6 Valency = 8 - 6 = 2
(iii) N 2.5 Valency = 8 - 5 = 3
Groups
A group is a vertical column of elements in the periodic table
The position of an element in the periodic table is determined by the number of electrons in the outer most shell.
There are eight groups on the periodic table
Groups are labeled with roman numerals i.e. groups I, II, III, IV, V, VI, VII with the final group labeled O
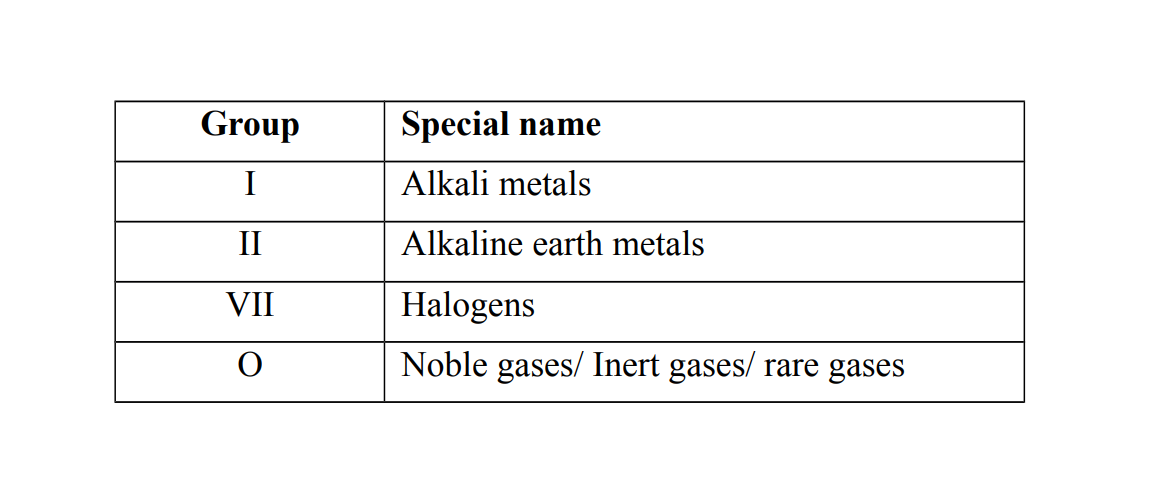
Some groups have special names
Example 3
Progression of properties down the group
As we move from top to bottom (down the group)
- The metallic nature of elements increases.
- The atomic number increases.
- Elements in the same group have similar chemical properties.
- Elements in the same group have the same number of electrons in the outer most shell.
- For non-metals, the group number is equal to eight minus the valence.
- For metals, the group number is equal to the valence.
Position of hydrogen in the periodic table
Hydrogen is placed between group I and group VII because it behaves like group I and group VII elements i.e. it can lose or gain a single electron.
Zig-zag diagonal line
The zig-zag diagonal line in the periodic table divides metallic elements from non-metallic elements.
Elements near the line are called metalloids
Metalloids have the characteristics of both metals and non – metals
Metalloids are also called semi-metals
The Periodic table Group properties
Group I elements
They are called alkali metals because they react with water to form alkaline solutions. (Alkalis) the Alternative term: Alkali metals
Examples of group I elements

Occurrence of group I elements
Group I elements do not occur naturally as free elements because they are very reactive. They are found in compounds e.g. rock salt (impure sodium chloride) which is a good source of sodium.
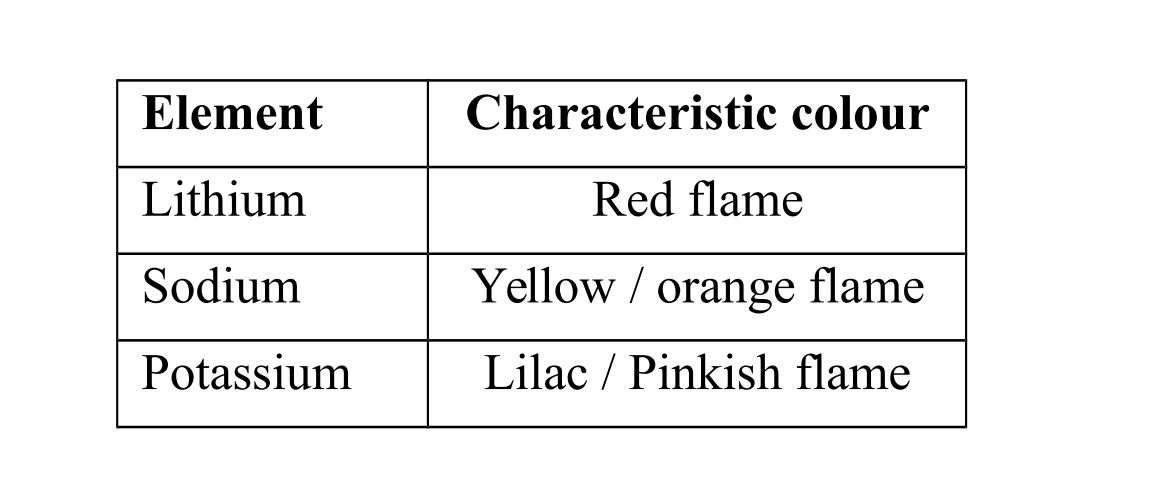
Identification of group I elements
Group I elements and their compounds give characteristic colours in a flame.
Storage of group I elements
Group I element are stored under oil to prevent them from reacting with atmospheric air or water.
Physical properties of group I elements
- They are soft metals which can be cut with a knife.
- They are good conductors of heat and electricity.
- They have low densities and hence float on water as they react with it. Their densities increase as you go down the group.
- They have low melting and boiling points. Their melting points decreases as you go down the group.
Chemical properties of group I elements
1. They have a single electron in their outer most shells.
e.g (a) Li 2.1 (b) Na 2.8.1 (c) K 2.8.8.1
2. They lose their single electrons in their outer most shells to form ions with 1+ charge.
Group VII elements
They are called Halogens because they react with group I elements to form salts.
The Alternative term: Halogens. The term halogen means salt former
Examples of group VII elements
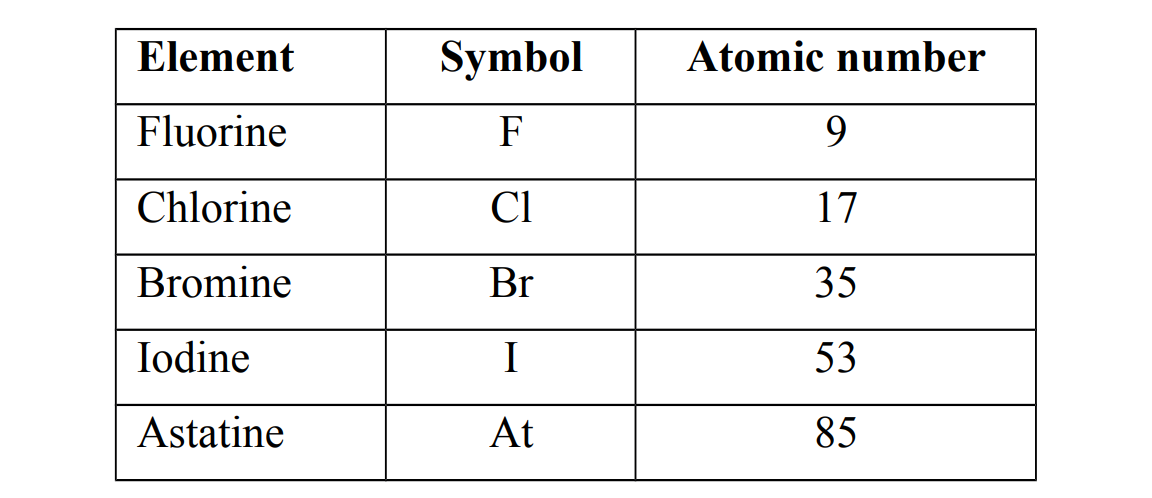
Occurrence of group VII elements
Group VII elements do not occur naturally in a free state, instead they exist as diatomic molecules meaning two atoms chemically combined.
Physical Properties of Group VII Elements
1. They exist as diatomic covalent molecules (meaning two atoms chemically combined)
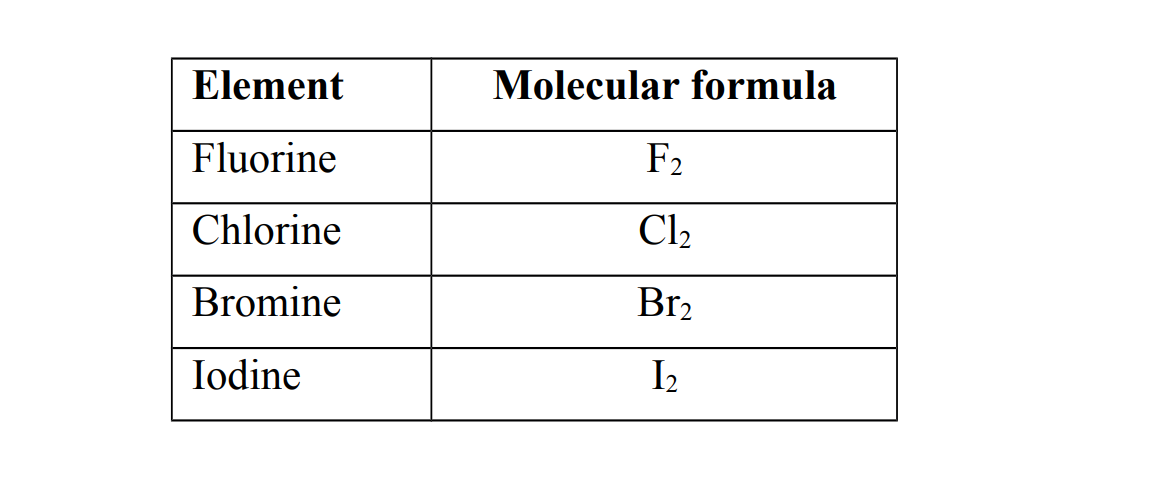
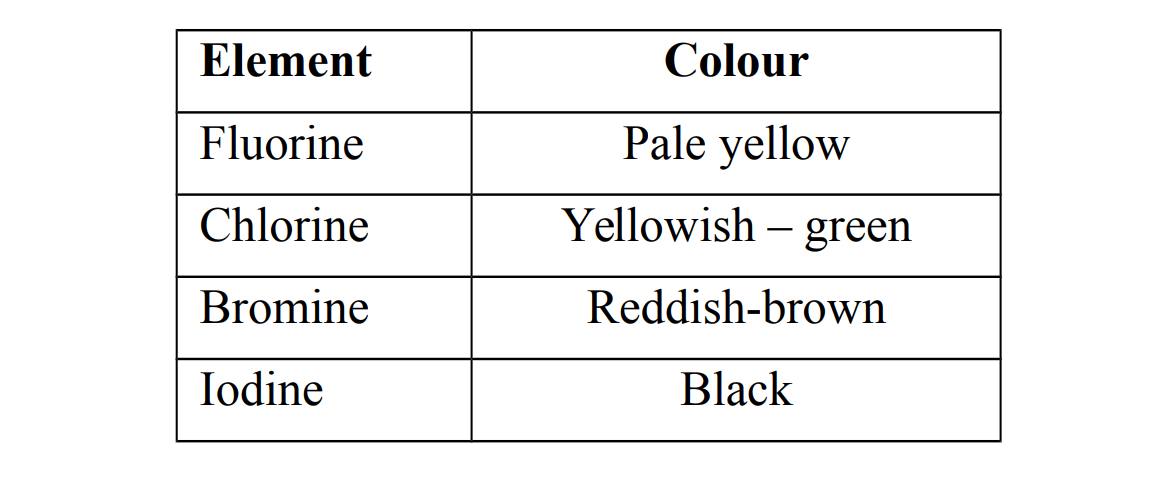
2. They exist as coloured,non- metallic elements
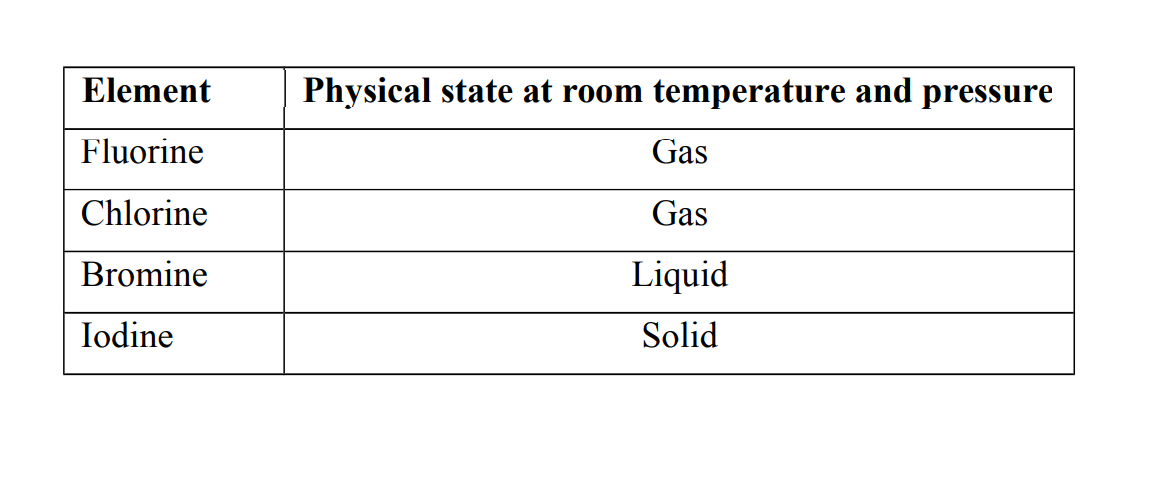
3. They show a gradual change in their physical states at room temperature and pressure.
4. Their melting and boiling points increases as you go down the group
5. Their densities increases as you go down the group.
6. Their compounds can either be ionic or covalent. If they combine with a metal the compound is ionic and if they combine with another nonmetal the compound is covalent.
Chemical properties of group VII elements
1. They all have seven electrons in their outer shells and hence have similar chemical properties. E.g (a) F 2.7 (b) Cl 2.8.7 (c) Br 2.8.18.7
2. They gain a single electron to form ions with 1- charge
3. They are oxidizing agents because they accept /gain electrons.
4. They react with group I elements to form salts
Uses of group VII elements
- Fluorine is used in fluoride tooth paste to help prevent tooth decay.
- Chlorine is used to sterilize drinking water because it kills harmful micro-organisms.
- Iodine is used as an additive in table salt to prevent goiter in human beings.
Harmful Effects of Group VII Elements
Compounds of group VII elements are known to be responsible for the depletion of the Ozone layer.
Note. Ozone is an allotrope of oxygen made up of three oxygen atoms ()
Group O elements
They are called noble gases or inert gases or rare gases because they are chemically unreactive and therefore do not form compounds. Alternative term: Noble gases / Inert gases / Rare gases.
Group O elements are chemically unreactive because they have full outer most electron shells. Their outer most shells are completely filled.
Group O elements are gases at room temperature and pressure. Group O elements exist as unreactive monatomic elements with very low melting and boiling points. They consist of single atoms.
Examples of Group O elements.
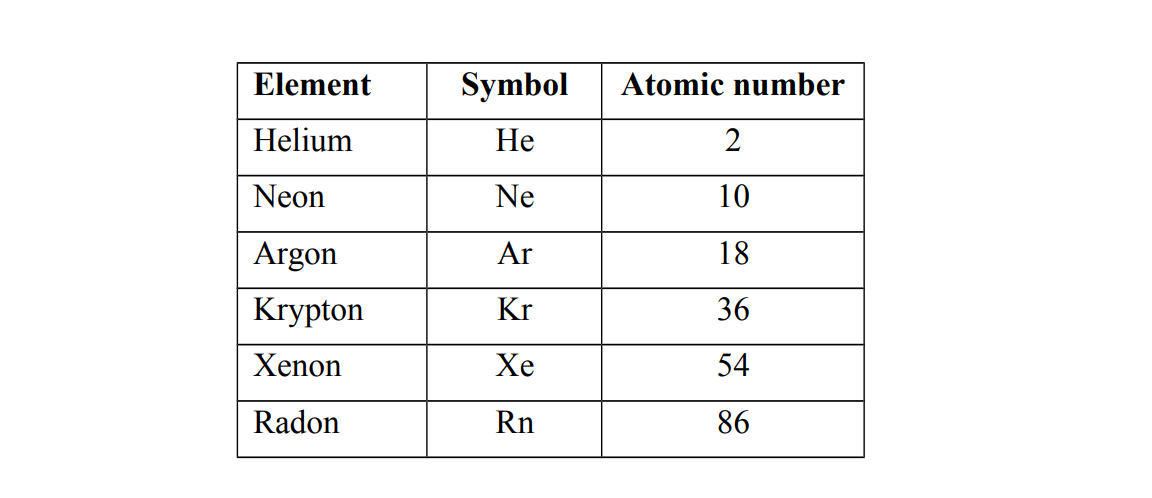
Group O elements have eight electrons in the outer most shell except for helium which has only two electrons. e.g (a) He 2 (b) Ne 2.8 (c) Ar 2.8.8 (d) Kr 2.8.18.8
Uses of group O elements
- Helium is used to fill weather balloons because of low density.
- Neon is used to fill coloured glowing tubes used in advertisements because it glows red hot in an electric current.
- Argon is used to fill light bulbs to provide an inert atmosphere to prevent the oxidation of the filament
Transition elements
Transition elements are found in the centre block of the periodic table. They are found between group II and III of the periodic table and through periods 4 and 6 They are all metals.
Examples of transition elements
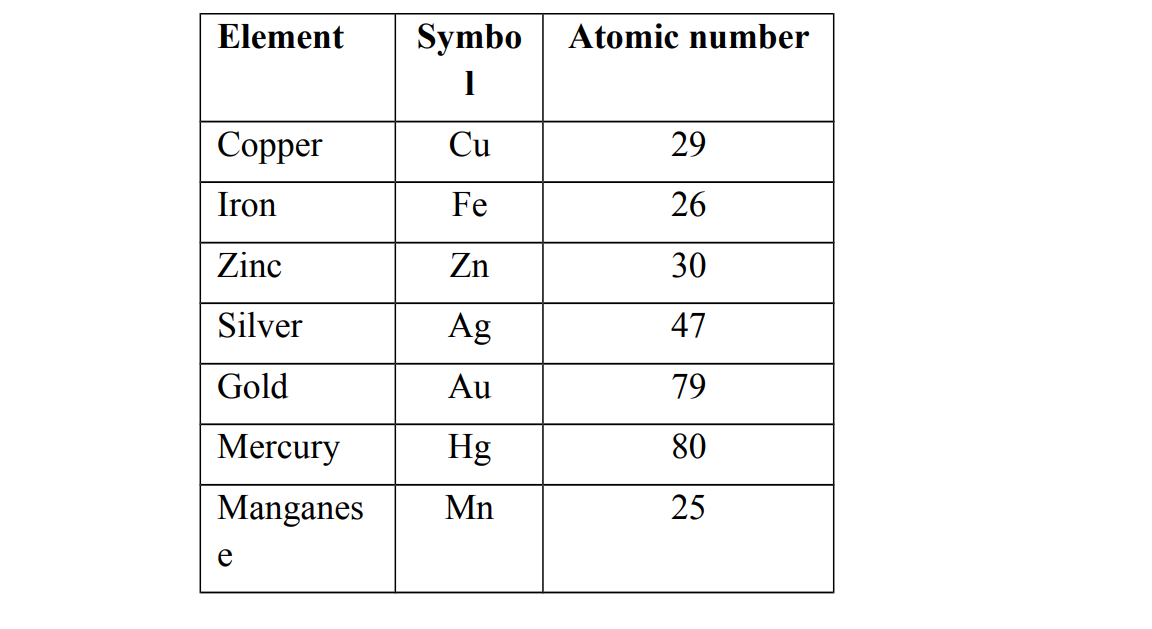
Physical properties of transition elements
- They have high densities
- They have high melting and boiling points
- They are good conductors of heat and electricity
- They are solids at room temperature and pressure except mercury which is a liquid
- They are ductile i.e. they can be drawn into wires
- They are malleable i.e. they can be hammered into thin sheets.
Chemical properties of transition elements
- They are catalysts
- They have variable Valencies and form positively charged ions.
- They form coloured compounds depending on the valence used. e.g (a) Copper (II) compounds are blue (b) Iron (II) compounds are pale green (c) Iron (III) compounds are reddish brown
- They are reducing agents because they lose electrons.
Uses of transition elements
- They are used to make electric cables because they are good conductors of electricity.
- They are used to make pots and pans because they are good conductors of heat.
- They are used to make alloys. e.g (a) Brass is an alloy of copper and Zinc (b) Bronze is an alloy of copper and tin
- They are used as catalysts in the industry to speed up reactions. e.g (a) Iron is used as a catalyst in Haber process. (b) Nickel is used as a catalyst in the hydrogenation of oil to make margarine (c) Platinum is used as a catalyst in contact process.