Alcohols
Alternative term: Alkanols
General molecular formula: OH where n = 1, 2, 3, 4
Functional group: hydroxyl group, ─ OH . They end with anol. They are not hydrocarbons because they contain oxygen
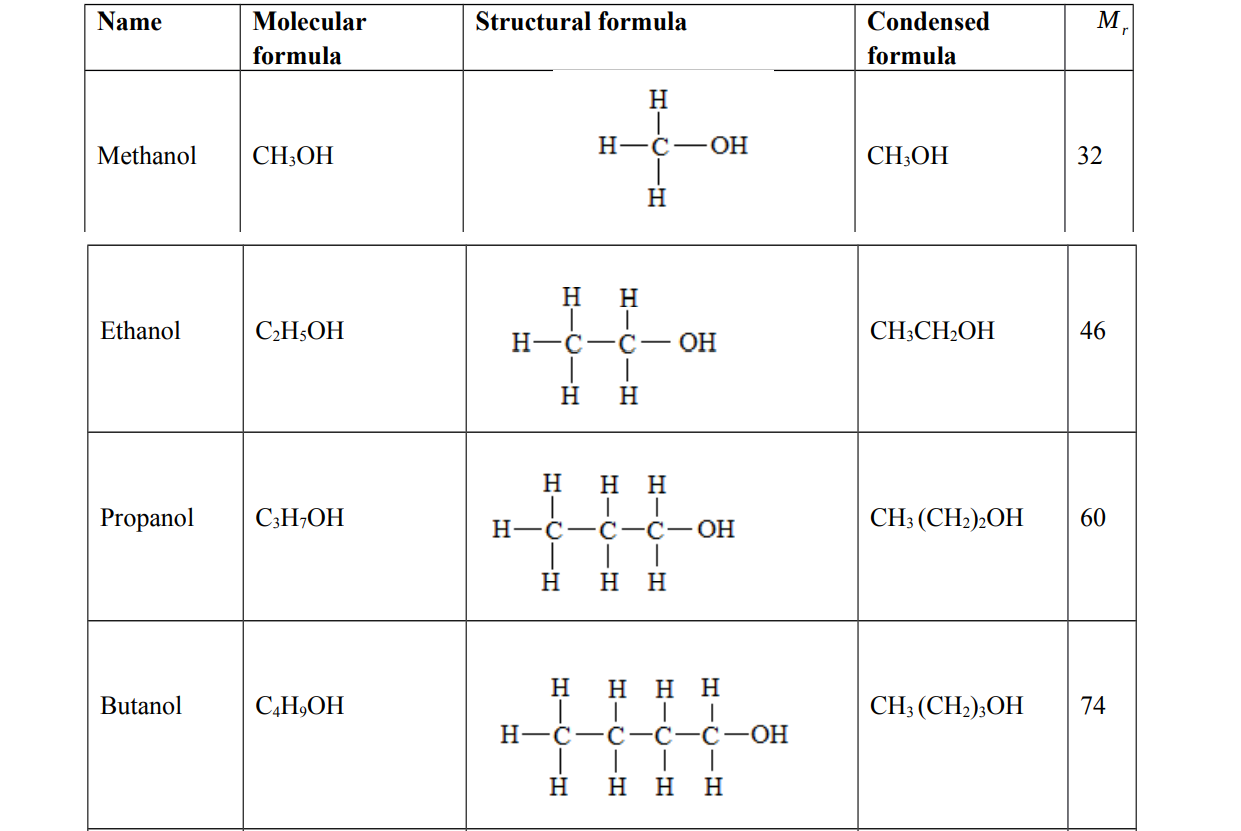
Examples of alcohols
Preparation of ethanol
There are two methods of preparing ethanol.
1. Hydration of etheneEthanol can be prepared when ethene reacts with steam
C2H4(g) + H2O (g) → C2H5OH(l)
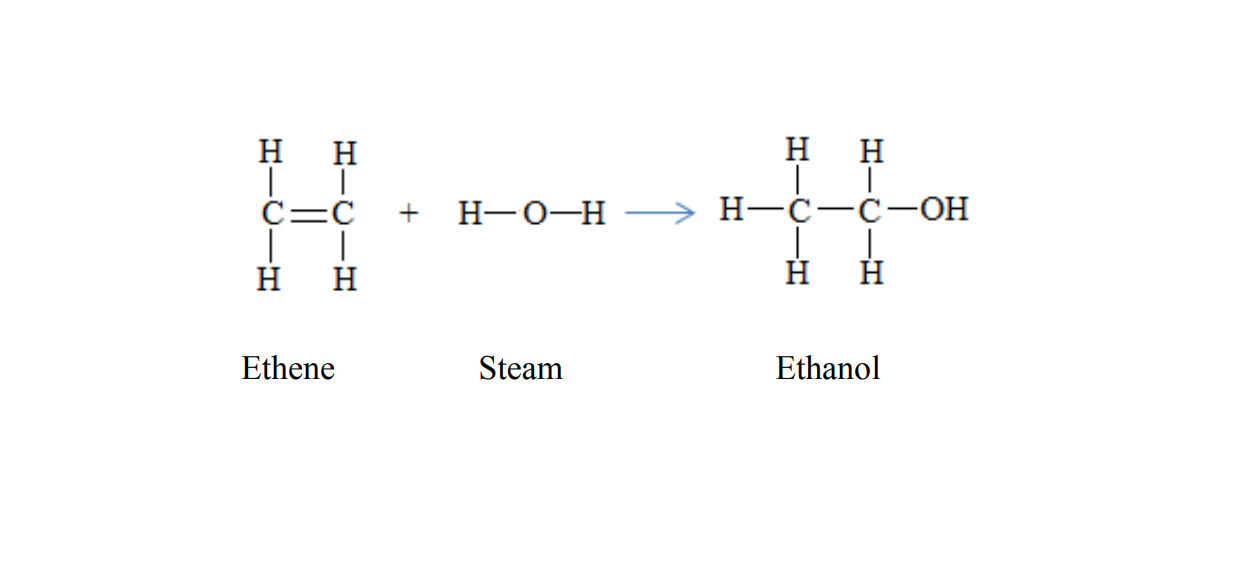
Temperature: 300 degree Celsius
Catalyst: Phosphoric acid,
Pressure: 65 atmospheres
2. Fermentation of sugarsFermentation is the decomposition of sugars using enzymes in yeast to produce ethanol and carbon dioxide. Sugars (glucose) is mixed with water and yeast and allowed to react for a few days in the absence of air.
Glucose → ethanol + carbon dioxideC6 H12O6 → C2 H5 OH + CO2
Conditions for fermentation
Optimum temperature: 37 degree Celsius
Catalyst (enzyme): Zymase
Note Fermentation should take place in the absence of air (oxygen) to prevent oxidation of ethanol to ethanoic acid.
If the temperature goes above 40 degree Celsius , the enzymes in yeast which catalyze the reaction becomes denatured.
Physical properties of alcohols
- They are colourless inflammable liquids
- Their boiling points increases as the carbon chain increases
- Their solubilities in water decreases as the number of carbon chain increases
Chemical properties of alcohols
1. Combustion
Alcohols burn in air (react with oxygen) to form carbon dioxide and water.
Ethanol + oxygen → carbon dioxide + water
C2 H5OH + 3O2 → 2CO2 + 3 H2O
The reaction is exothermic. It gives out a lot of heat energy
2. Oxidation
Oxidation is the addition of oxygen to a substance.
(a) Ethanol can be oxidized to ethanoic acid by bacteria in the air
C2 H5 OH + O2 → CH3 COOH + H2 O
(b) Ethanoic acid can also be formed by using an oxidizing agent such as acidified potassium per manganate and potassium dichromate (VI)
C2 H5 OH + 2[O] → CH3 COOH + H2 O
The oxygen is from the oxidizing agent.
When ethanol reduces potassium permanganate, the reaction is indicated by the colour change from purple to colourless on mixing.
When ethanol reduces potassium dichromate, the reaction is indicated by the colour change from orange to green on mixing.
Uses of ethanol
- Used as a component in beer and wines
- Used as a solvent
- Used in making methylated spirit
- Used as a fuel
- Used in the preservation and sterilization of food