Macromolecules (Polymers)
Macromolecules are giant molecules formed by joining smaller units called monomers. Alternative term: Polymers.
Macromolecules are produced by the process called polymerization.
Polymerization
Polymerization is the joining up of smaller units called monomers to form larger molecules called polymers.
Types of macromolecules
There are two types of macromolecules.
- Synthetic macromolecules
- Natural macromolecules
Synthetic macromolecules
Synthetic polymers are man-made structures. Alternative term: Synthetic or artificial polymers
They are divided into two categories:
- Addition polymers
- Condensation polymers
Addition polymers
Addition polymers are polymers formed from smaller identical unsaturated monomers No other product is formed apart from the polymer Addition polymers are formed by the process called addition polymerization.
Addition polymerization is polymerization where the polymer has the empirical formula as the monomer.
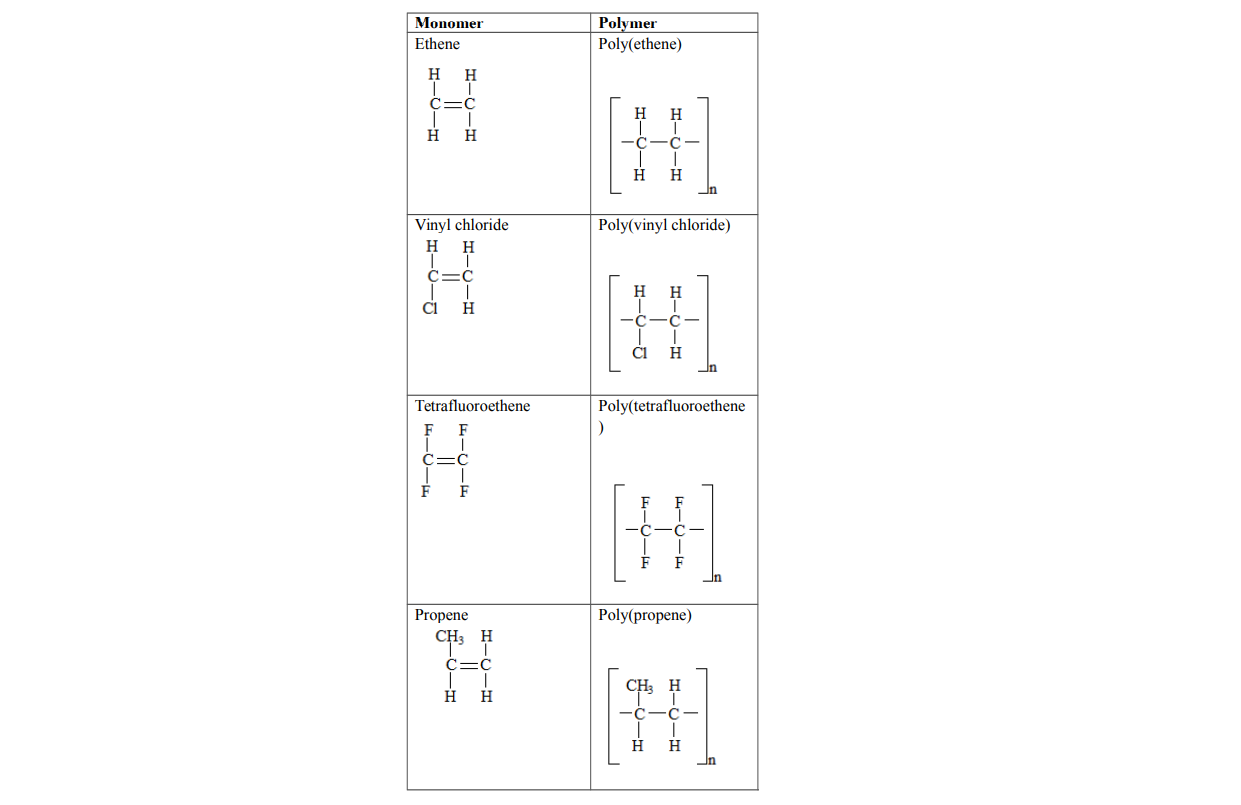
Examples of addition polymers
1. PolyethenePolyethene is formed when ethene molecules combine
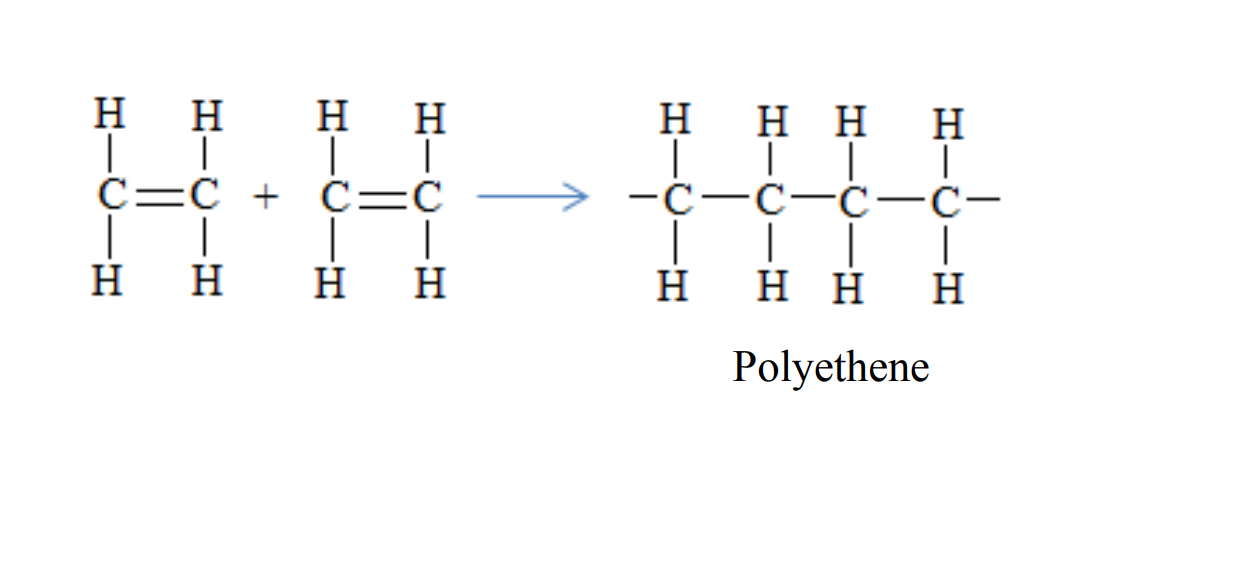
Uses of Polyethene
- Used in making plastics bags
- Used in making squeezing bottles
Polyvinyl chloride is formed when vinyl chloride molecules combine
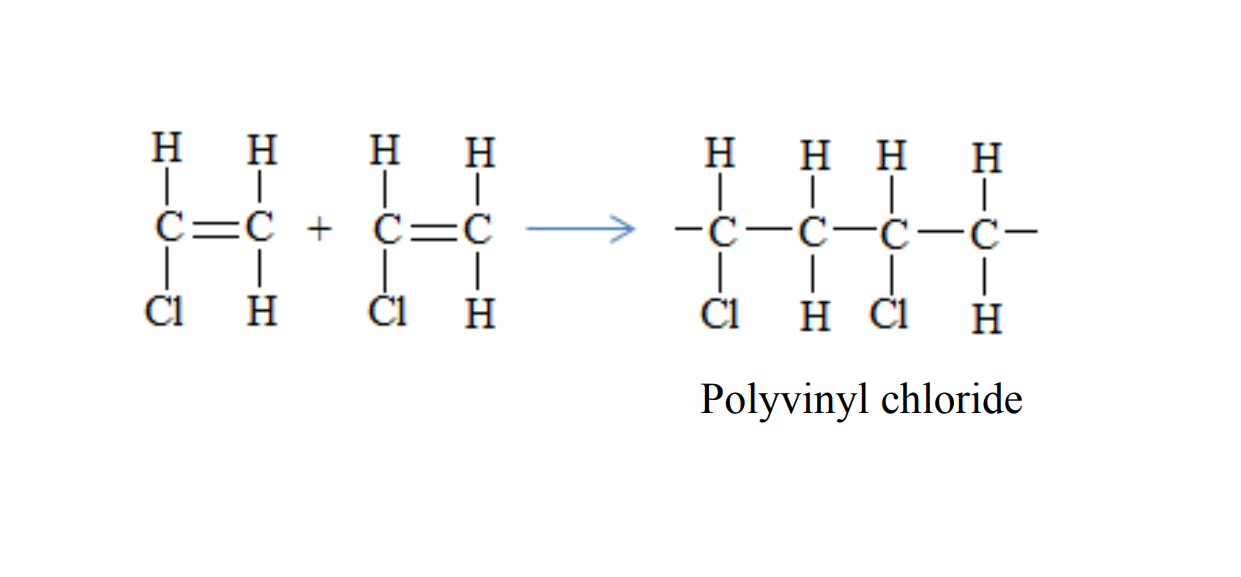
Uses of polyvinyl chloride
- Used in making PVC paints
- Used in making electrical insulators, records, seat covers, rain coats
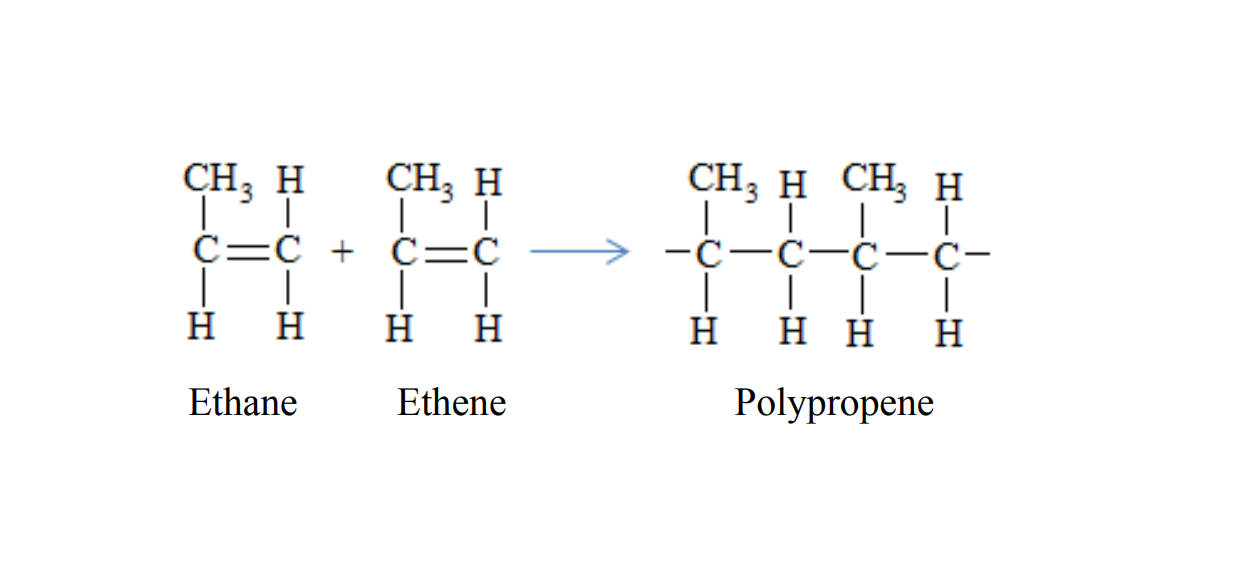
Polypropene is formed when propene molecules combine
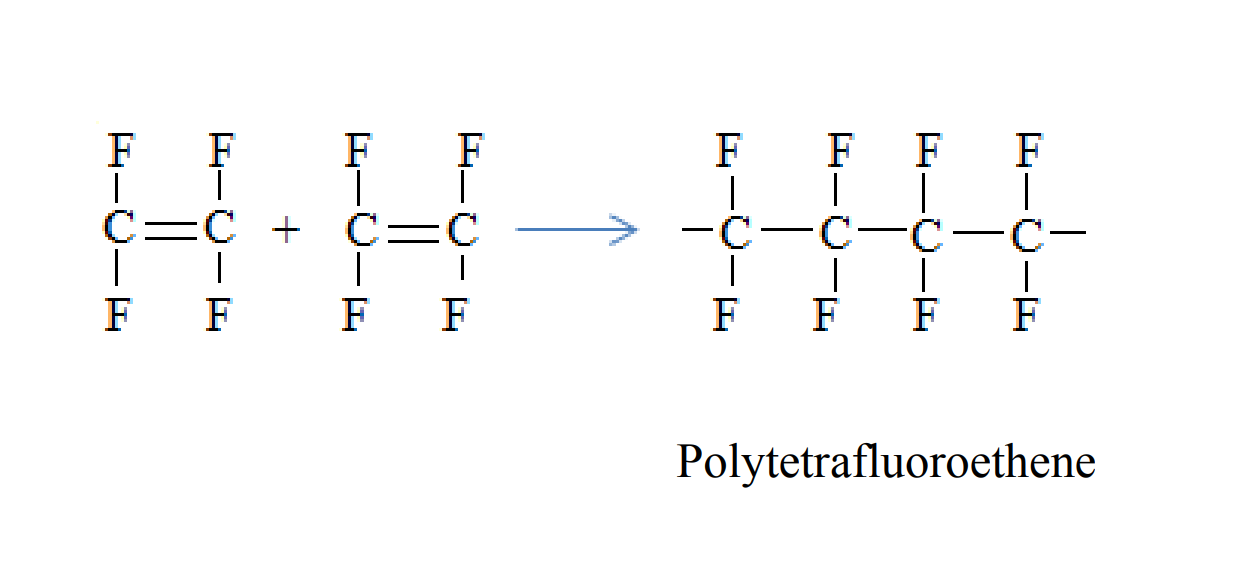
Polytetrafluoroethene is formed when tetrafluoroethene molecules combine
Condensation polymers
Some man-made polymers are formed by condensation polymerization.
Condensation polymerization involves two smaller units which combine to form a larger molecule with the elimination of the water molecule.
Condensation polymers do not have the same empirical formula as the monomers.
Examples of condensation polymers
- Nylon
- Terylene
Nylon is a typical polyamide with amide linkages. A polyamide is a polymer containing many amide linkages. Monomers: Diamine and Dicarboxylic acid
The structure of nylon is similar to that of protein
Uses of nylon
- Making tough bearing
- Making clothing
- Making ropes
- Making bristle for brushes
Terylene is typical polyester with ester linkages.
A Polyester is a polymer containing many ester linkages Monomers: Diol and Dicarboxylic acid
The structure of Terylene is similar to that of fats.
Uses of Terylene
- Making tents and sails.
- Making clothing
Advantages of synthetic polymers
- They are durable. They do not rust, corrode or decay
- They are lighter than steel, wood or stone
- They are thermal and electrical insulators
- They are not expensive. They are produced as by-products of oil refining
- They are flexible in use
Disadvantages of synthetic polymers
- They are non-biodegradable. This means they cannot be decomposed by bacteria.
- Non-biodegrability makes the disposal of plastics difficult and this result in pollution problems.
- Plastics burn easily and may produce poisonous gases on combustion. They need to be coated with fire retardants to reduce the risk of fire.
Reasons for recycling plastics
- They are difficult to dispose of: Plastic bags do not rot when they are thrown away, so they pollute the environment
- When some plastics burn, they produce harmful gases: For example polyvinylchloride (PVC) gives off fumes of hydrogen chloride when it burns. This would form hydrochloric acid in the eyes and throat.
Natural macromolecules occur in living organisms. Alternative term: Natural polymers
Examples of natural macromolecules
- Proteins
- Fats
- Carbohydrates
Proteins are made by condensation polymerization. They are condensation polymers
They are poly amides like nylon because they contain the amide linkages. Protein hydrolysis results in amino acids
FatsFats are complex esters formed from fatty acids and glycerol. Fats have the structure similar to Terylene and poly carbonates
Fat hydrolysis results in fatty acids and glycerol.
CarbohydratesCarbohydrates are sugars which include starch and cellulose. Carbohydrates are formed from simple sugars like glucose