Carbon and carbonates
Allotropes of carbon
Allotopes are elements in different physical forms but in the same state Allotropy is the existence of an element in different physical forms but in the same state Carbon exists in the form of graphite, diamond and amorphous Grapphite and diamond are allotropes of carbon.
Graphite
Structure of graphite
Graphite is a soft, black, crystalline form of carbon that is a fair conductor of electricity. It is made up of flat sheets of carbon atoms
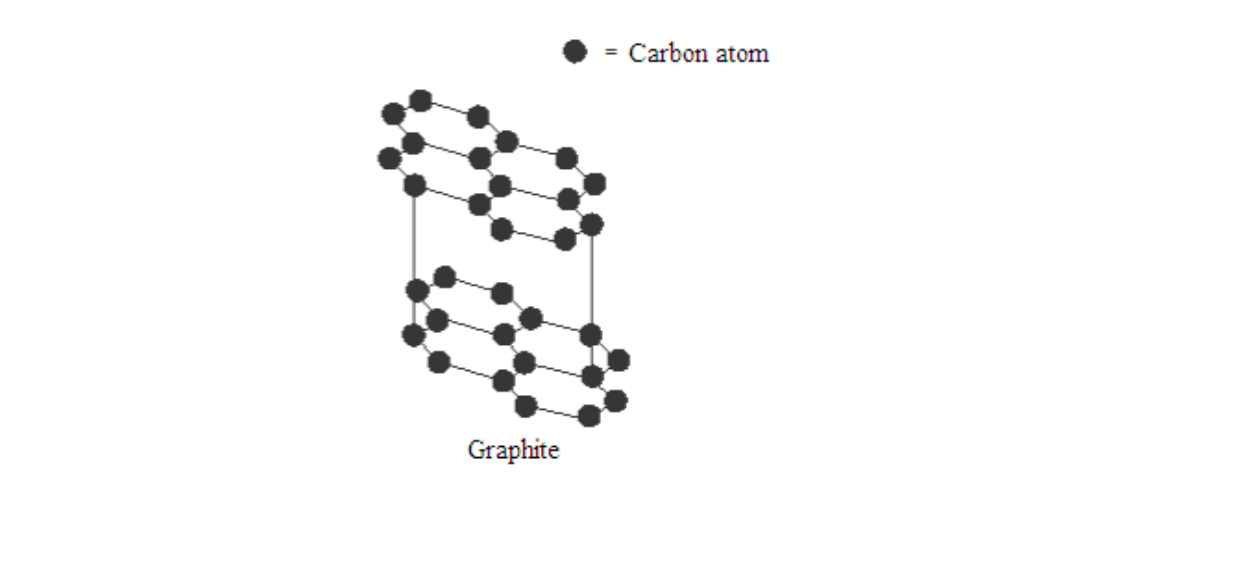
Each carbon atom is boneded to three others while the fourth electron is delocalised. Since each carbon atom forms covalent bonds to three other, this gives rings of six atoms that join to make flat sheets
The sheet of atoms lie on top of each other, held together by weak forces
Physical properties of graphite
- It is a good conductor of electricity because of free moving electrons in between the layers of carbon atoms
- It is soft and slippery. This is because the sheets of atoms can slide over each other easily.
- It writes well on paper
- It is has a density of 2.22g/cm3
- It has a high melting point. This is because the strong bonds between the carbon atoms within a layer make graphit difficult to pull apart in the direction of the layer.
Uses of graphite
- It is used as a lubricant because the layer of carbon atoms slide over each other easily
- It is used as an electrode in electrolysis
- It is used in making the “lead” for pencils. This is because it leaves a grey streak or when it is drawn across a sheet of paper
Diamond
Structure of diamond
Diamond is a colourless, crystalline solid with an extremely high density. It is a giant structure of carbon atoms
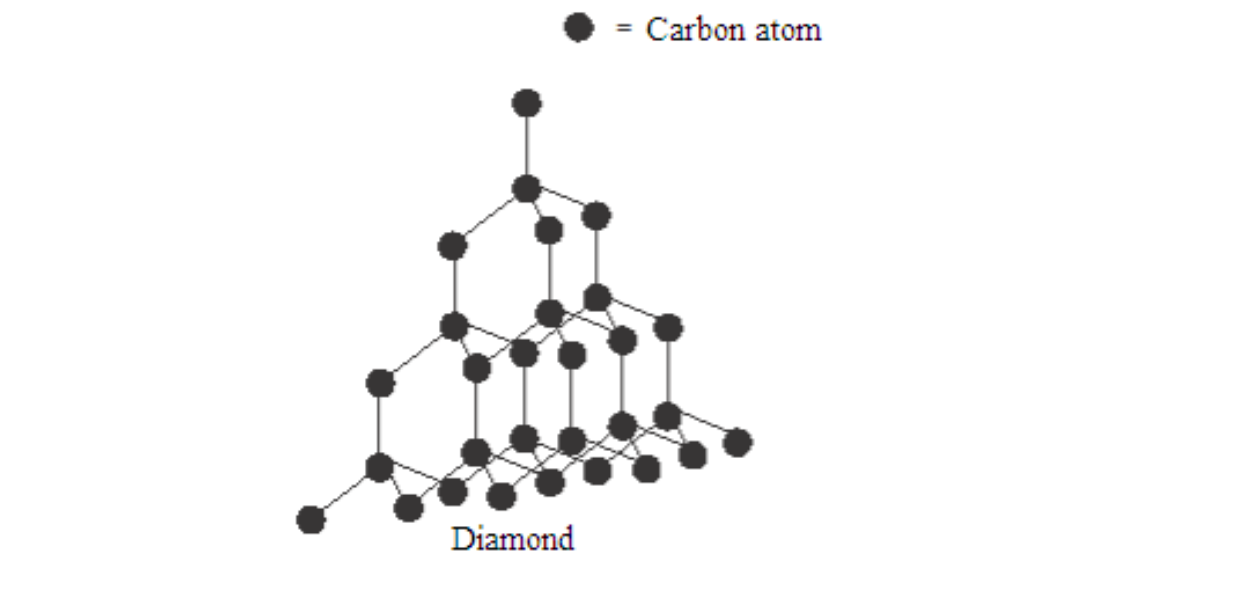
Each carbon atom shares electrons with each of its four nearest neighbours, thus forming four covalent bonds.
In addition, each carbon atom is imagined to be at the centre of the tetrahedron surrounded by four other carbon atoms whose centres are at the corners of the tetrahedron.
Physical properties of diamond
- It is very hard - the hardest substance known. It has a very high melting point of about 3700oC because each atom is held in place by four strong bonds.
- It is colourless and transparent with a dazzzling brilliant lustre.
- It has a density of 3.5g/cm3.
- It does not conduct electricity because there are no ions or free electrons in it to carry charge.
Uses of diamond
- It is uesd for cutting tools and drilling devices
- It is used for cutting glass
Amorphous carbon
Amorphous carbon such as coal and charcoal is porous and easily absorbs pigments from solutions e.g in the refining of white spoon sugar
Charcoal is used to absorb the brown colour from brown sugar which is then turned white
Chemical properties of carbon
All the forms of carbon react with oxygen to form carbon dioxide
Carbonates, oxides and hydroxides
Calcium carbonate
Chemical formula: CaCO3
Calcium carbonate is a white solid which is insoluble in water In nature, calcium carbonate exists as limestone, marble, chalk and calcite When strongly heated, calcium carbonate decomposes to form calcium oxide and carbon dioxide
CaCO3(s) → CaO(s) + CO2(g)
Uses of calcium carbonate
- It is used in the manufacture of cement
- It is used for making glass
- It is used to remove impurities like silica as slag in the blast furnace
Calcium oxide
Chemical formula: CaO
Special name: Quick lime
Calcium oxide reacts vigorously with water to form calcium hydroxide and a lot of heat energy
CaO(s) + H2O(l) → Ca(OH)2(aq) + Heat energy
Calcium hydroxide
Chemical formula: Ca(HO)2
Special name: Slaked lime or lime water
Calcium hydroxide glows brightly at high temperatures Calcium hydroxide turns milky or cloudy when carbon dioxide is passed through it and a white precipitate (suspension) of calcium carbonate is formed
Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l)
When excess carbon dioxide gas is passed through for some time, the precipitate disappears and a clear solution of calcium hydrogen carbonate is formed
CaCO3(s) + H2O(l) + CO2(g) → Ca(HCO3)2(aq)
Uses of calcium oxide and calcium hydroxide
- They are used in treating acidic soils on the farm
- They are used in the neutralization of acidic industrial waste products before discharging them into rivers and lakes
- Calcium hydroxide is used as plaster of paris for broken arms and legs
- Calcium oxide is used as lining in the blast furnace.
- Calcium oxide is used to remove silica impurities in the extraction of iron.
- Calcium oxide is used as a drying agent especially for ammonia gas.